We all know what water is. And what rock is. The difference is crystal clear. Well, here on Earth it is.
But on other worlds? The difference might not be so clear.
Among the hundreds of billions of stars in the Milky Way are who-knows-how-many exoplanets. Some number of them will be ocean worlds, completely covered in water with little to no land-masses. And most of them will likely be much larger than Earth. These planets can host oceans up to 1,000 km thick, according to scientists, and all that water is pressing down on a rocky mantle.
Scientists have a bunch of questions about these type of planets. Could life develop on these worlds? If so, what kind of life? Could this type of world even support life, or is exposed land a critical part of a world’s biogeochemistry, like here on Earth?
A team of researchers led by Arizona State University wanted to do more than just think about them. In an effort to better understand these worlds, and to make some progress on these questions, they recreated a water-world in their lab. Sort of.

The results of their work were just published in the Proceedings of the National Academy of Sciences of the USA. The paper’s title is “Large H2O solubility in dense silica and its implications for the interiors of water-rich planets.” Lead author is Dan Shim, associate professor at ASU, and head of the University’s Lab for Earth and Planetary Materials.
The team of researchers used the Advanced Photon Source lab at the Department of Energy’s Argonne National Laboratory. That may seem incongruous—using an x-ray lab to study planets—but it worked. As lead author Dan Shim said in a press release, “People hardly think about astrophysics when talking about an X-ray facility. But we can use a facility like the APS to understand an object too distant for us to see.”
“If you were to build a planet with water and rock, you would assume that the water forms a layer above rock.”
Dan Shim, Lead Author, Arizona State University
In the lab, the researchers re-created the extreme properties of large ocean worlds. These planets can have mantles of rock, with vast amounts of water above them, pressing down on them with intense pressure. That crushing pressure also creates intense heat.
“Determining the geology of exoplanets is tough, since we can’t use telescopes or send rovers to their surfaces,” Shim said. “So we try to simulate the geology in the lab.”
The team placed samples of silica, or silicon dioxide, in between special diamonds in a device called a diamond anvil cell. They then compressed the samples to extremely high pressures, simulating the situation that rocky mantles find themselves in on large ocean planets. Then, they subjected the samples to infrared lasers.
“We can raise the pressure up to multiple millions of atmospheres,” said Yue Meng, a physicist in Argonne’s X-ray Science Division and a co-author on the paper. Meng was one of the main designers of the techniques used at HPCAT, which specializes in high-pressure, high-temperature experiments.
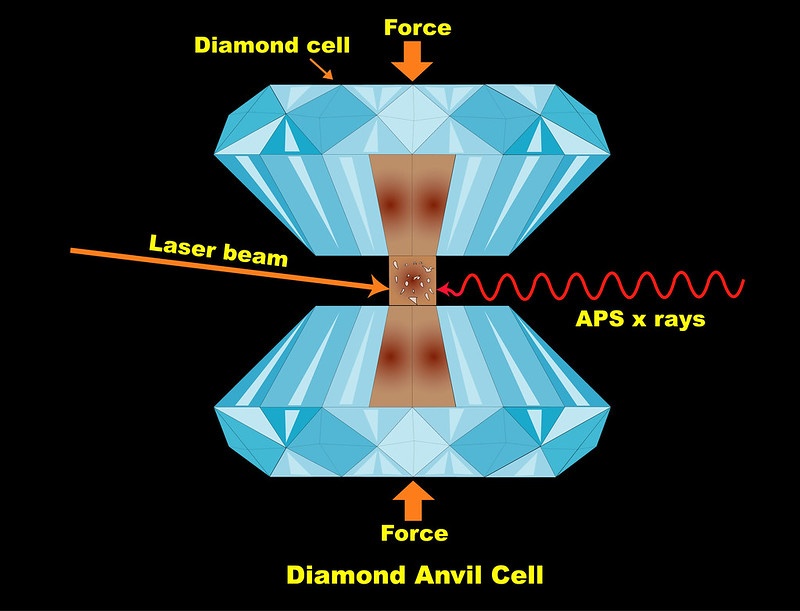
“The APS is one of the few places in the world where you can conduct this kind of cutting-edge research,” she said. “The beamline scientists, technicians and engineers make this research possible.”
Though we don’t know a lot of the specific characteristics of large ocean worlds, scientists can calculate their pressures. Once the samples were brought up to ocean world pressures, they were subjected to the lasers, which heat the sample up to temperatures found at the rocky mantles of these planets, under all that water. Those pressures vary quite a bit depending on things like the age of the planet, and the amount of radioactive decay heating. So the team calculated a range of temperatures for their experiments.
“We can bring the sample up to thousands of degrees Fahrenheit,” said Vitali Prakapenka, a beamline scientist at GSECARS, a research professor at the University of Chicago and a co-author on the paper. “We have two high power lasers that shine on the sample from both sides precisely aligned with an ultra-bright APS X-ray probe and temperature measurements along the optical paths with a sub-micron accuracy.”
So, what did the researchers find when they subjected silicon dioxide samples to these extreme pressure and temperatures?
“Before we believed that there was a separation between rock and water, but based on these studies, there is no sharp boundary.”
Vitali Prakapenka, study Co-Author, beamline scientist, And Research Professor, University of Chicago
When they subjected the samples to high temperatures and to pressures of 30 gigapascals, which is about 300,000 times more pressure than on Earth’s surface level, the water and the silica merged.
They had found a new transitional phase between silica and water, indicating that the boundary between water and rock on these exoplanets is not as solid as it is here on Earth. This startling discovery will change how scientists think about exoplanets, and how they model them.
“If you were to build a planet with water and rock, you would assume that the water forms a layer above rock,” he said. “What we found is that is not necessarily true. With enough heat and pressure, the boundary between rock and water becomes fuzzy.”
That means it’s time to update our exoplanet models.
“The main point is that it tells the people modeling the structure of these planets that the composition is more complicated than we thought,” said Vitali Prakapenka, a beamline scientist at GSECARS, a research professor at the University of Chicago and a co-author on the paper. “Before we believed that there was a separation between rock and water, but based on these studies, there is no sharp boundary.”
In fact, this new material could dominate ocean worlds.
“Therefore, the physical properties of the new phases that we report here would be important for understanding dynamics, geochemical cycle, and dynamo generation in water-rich planets.”
From the Paper “Large H2O solubility in dense silica and its implications for the interiors of water-rich planets.”
“Our results suggest that the phases containing both hydrogen and lithophile elements could be the dominant materials in the interiors of water-rich planets,” the authors write in their study. “Even for fully layered cases, the large mutual solubility could make the boundary between rock and ice layers fuzzy. Therefore, the physical properties of the new phases that we report here would be important for understanding dynamics, geochemical cycle, and dynamo generation in water-rich planets.”
This is critical work. The way that water and rock interact is huge when it comes to life on Earth. So what will this discovery mean for the future?
“It’s a starting point to build the way chemistry works on these planets,” Shim said. “How water interacts with rock is important for life on Earth, and therefore, it is also important to understanding the type of life that might be on some of these worlds.”
More:
"difficult" - Google News
June 30, 2020 at 06:26AM
https://ift.tt/3dRlclX
Deep Down in Ocean Worlds, it's Difficult to Tell Where the Oceans End and the Rock Begins - Universe Today
"difficult" - Google News
https://ift.tt/2VWzYBO
https://ift.tt/3d5eskc
Bagikan Berita Ini














0 Response to "Deep Down in Ocean Worlds, it's Difficult to Tell Where the Oceans End and the Rock Begins - Universe Today"
Post a Comment